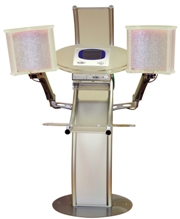

LED system (Light Emitting Diodes) for sin rejuvenation.
The photorejuvenation and photostimulation systems employ nowadays a new technique that take advantage of the characteristics of light. Indeed many of the wavelengths in the visible spectrum, allow to transmit therapeutic and aesthetic benefits on the living tissue.
In this regard was introduced the application of LED (Light Emitting Diodes) in medicine, where some specific wavelengths stimulate some important physical functions in the skin tissues.
With the LIGHT ACTIVE system we are using the characteristics of light through the use of the LED system conveniently programmed,
in order to reduce the effects of skin aging: these light emitting diodes trigger biochemical processes inside the epidermal cells. The positive effects on the cutaneous tissue are of two orders: collagen and elastin stimulation and inhibition of collagenase.
The LED-based technology trigger biochemical and non-ablative effects and represent a new type of phototherapy,
born in the recent developments and studies in the laser industry.
In contrast to lasers, pulse light or radiofrequency, the LED on the LIGHT ACTIVE device release low-intensity energy, without thermal effects and without ablation of the skin surface. The application times are fast and can be treated all skin types, including dark skins.
The LIGHT ACTIVE software emit two different wavelengths (orange and red) that can be used individually or in combination to amplify the benefits. The inner microprocessor is able to emit one single wavelength and/or to emit both of them in sequence, depending on the specific cases detected by the operator.
The aim and the fundamental skill of the LED technology is the stimulation and the inhibition of some types of cells: indeed recent in vitro studies have shown how the wavelengths in the near-infrared stimulate the fibroblasts synthesis and at the same time encourage the production of endothelium growth factors.
MEDICAL DEVICES NOTICE
In accordance with the New guidelines of the Italian Ministry of Health of 28/03/2013, in relation to health advertising concerning medical devices, the user is advised that the information contained therein is addressed exclusively to professional operators.
CERTIFIED COMPANY
M&T Srl is certified according to the European directives UNI EN ISO 13485:2016
AESTHETIC DEVICES
